About InSure® ONE™

InSure® ONE™—Early detection tool for colorectal cancer and other gastrointestinal disorders that bleed
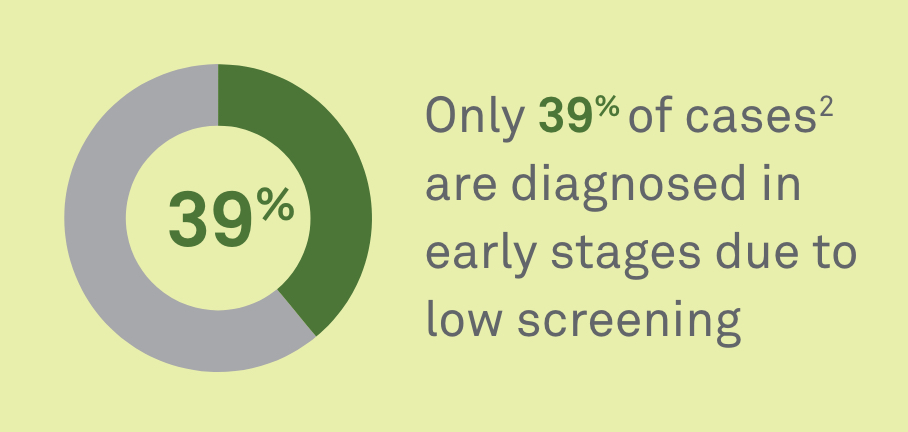
Colorectal cancer that is caught in the early stages has a 5-year survival rate of over 90%.1 Yet, only 39% of cases2 are diagnosed in early stages due to low screening rates. When disease spreads to distant organs, the 5-year survival rate drops to 11%.3
Fortunately, Quest Diagnostics can help you and your patients screen for signs of colorectal cancer and other gastrointestinal disorders that bleed.
InSure® ONE™ aids in the detection of lower gastrointestinal bleeding that may be caused by colorectal cancer or other lower gastrointestinal disorders.* Reach more patients with InSure ONE.
Accurate colorectal screening when used annually
Order InSure ONE in just 2 easy steps

Patient reminder program improves kit return rates
As a leader in colorectal cancer screening, we know that every kit returned could save a life by early detection. Our InSure ONE integrated reminder program helps encourage test compliance by sending reminder messages to patients to complete and return their collection kits.
Electronic orders for InSure ONE placed in Quanum® Lab Services Manager or your EMR interface are eligible to receive reminders.
Patient-friendly collection
The convenient water-based collection method uses unique, patented brush sampling that doesn’t require any stool collection or handling. There are no dietary or medicinal restrictions. Patients collect 2 water-based samples from a single bowel movement. This patient-friendly approach to testing is associated with up to 66% greater patient compliance.7

Please contact your Quest Diagnostics representative for instructions in additional languages.
How to get started
|
|
Start ordering InSure ONE kits using Quanum Lab Services Manager. If you don’t have a Quanum account, create one in less than 5 minutes by clicking here. If you would prefer to order kits over the phone, please call 1.866.MY.QUEST (1.866.697.8378) and state “supplies” upon connection for assistance. |
|
|
Search for test code 11290 for InSure ONE kits, where you will find all patient instructions for specimen collection and for returning completed test kits. |
|
|
Quest will ship the kits to your practice at no cost, based on usage and test completion. |
|
|
Distribute kits to average-risk patients aged 45+ during annual wellness visits. A completed requisition form from Quanum Lab Services Manager or your EMR should be printed and placed in the business reply envelope found inside the test kit envelope prior to the patient leaving the office. |
Not familiar with Quanum Lab Services Manager? You can sign up for an account at enroll.quanumsolutions.com/enrollment
Dramatic decrease in new colorectal cancer diagnoses during COVID-19

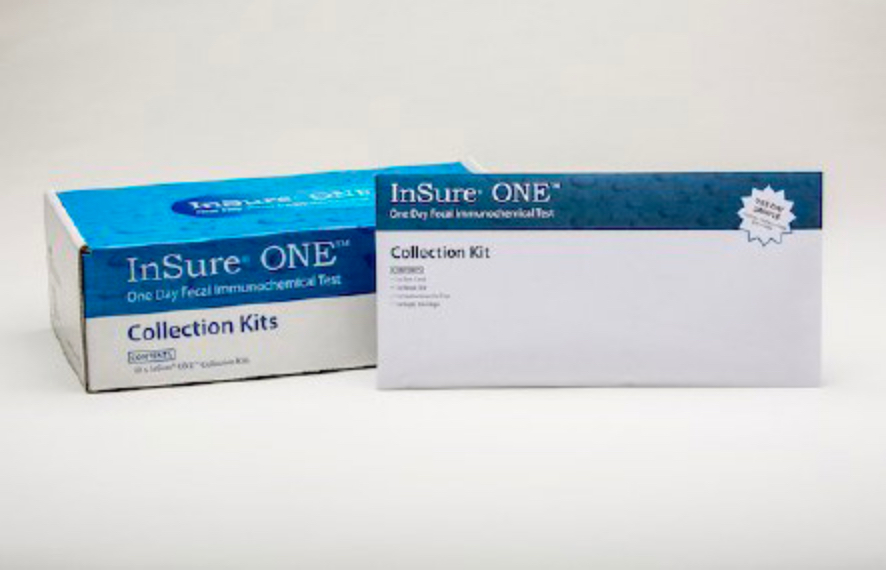
What’s in the kit
InSure® ONE™ comes in a patient-friendly kit that contains everything necessary to collect a water-based sample and package it for shipping in an enclosed self-mailing envelope.
The kit includes:
- Patient instructions for use
- A test card
- 2 long-handled brushes
- 2 waste bags
* InSure ONE is a fecal immunochemical test (FIT) that qualitatively detects human hemoglobin from blood in fecal samples. The samples will generally be collected by the test subject at home and the test developed at laboratories or professional offices. The InSure ONE test is used to aid in the detection of lower gastrointestinal bleeding.8
The CPT® codes provided are based on American Medical Association guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payer being billed.
Test codes may vary by location. Please contact your local laboratory for more information.
References
- Altekruse SF, Kosary CL, Krapcho M, et al (eds). SEER 18 2010–2016, All Races, Both Sexes by SEER Summary Stage 2000. Accessed July 31, 2020. https://seer.cancer.gov/statfacts/html/colorect.html
- American Cancer Society. Colorectal Cancer Facts & Figures 2017-2019. Published 2017. Accessed July 31, 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf
- InSure® ONE™. K170548 – Instructions for use. Quest Diagnostics; 2017.
- InSure® FIT. F002457 Fecal immunochemical test immunoassay for human hemoglobin in stool. Product instructions. Enterix; 2011.
- Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315(23):2564-2575. doi:10.1001/jama.2016.5989
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112(7):1016-1030. doi:10.1038/ajg.2017.174
- Cole SR, Young GP, Esterman A, Cadd B, Morcom J. A randomised trial of the impact of new faecal haemoglobin test technologies on population participation in screening for colorectal cancer. J Med Screen. 2003;10(3):117-122.
- Enterix, Inc. InSure ONE Fecal Immunochemical Test Instructions for Use. Published 2017.https://d1io3yog0oux5.cloudfront.net/clinicalgenomics/files/ pages/clinicalgenomics/db/839/description/InSureONE+Professional+IFU+press+ready.pdf
Image content features models and is intended for illustrative purposes only.