Because there’s more to monitoring
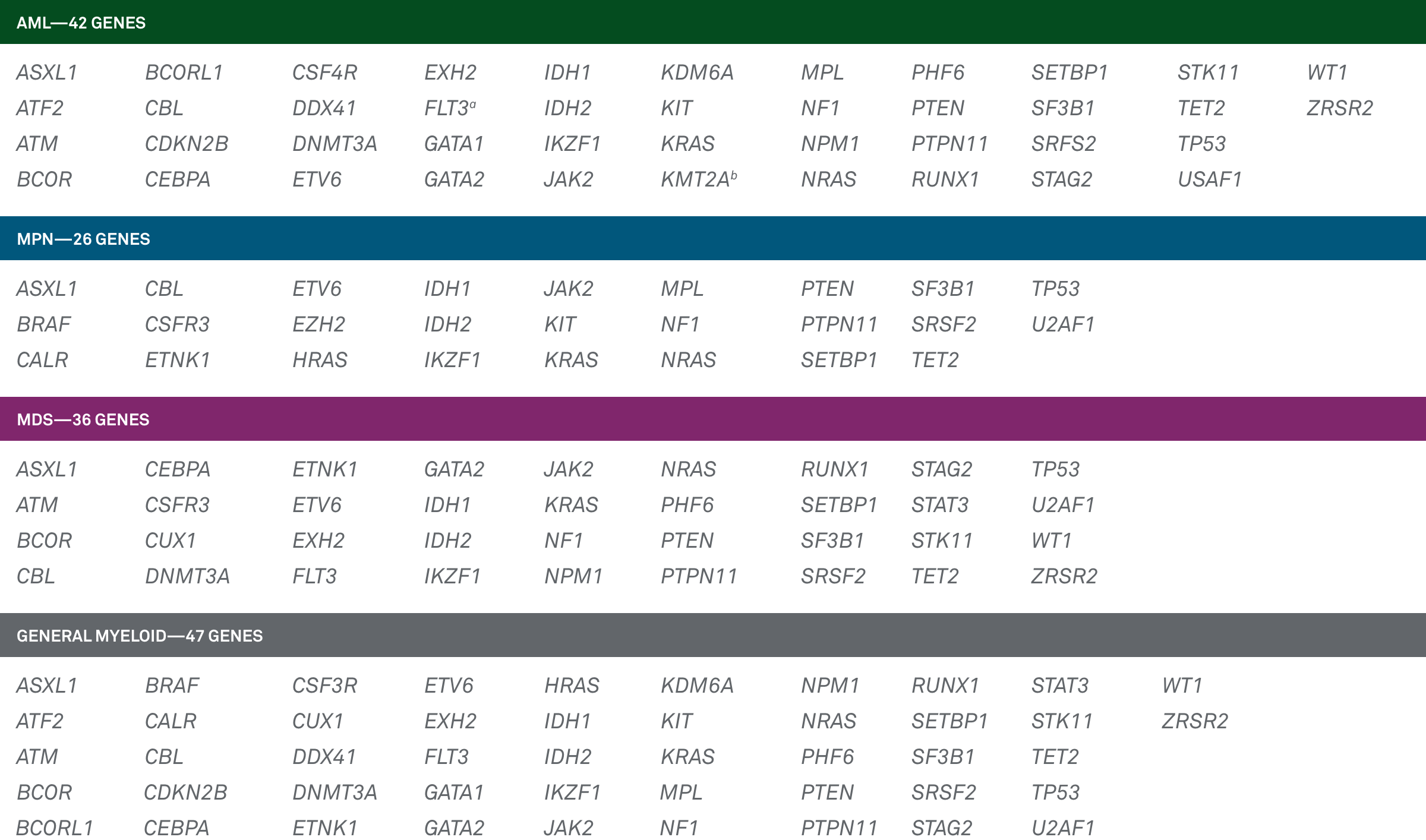
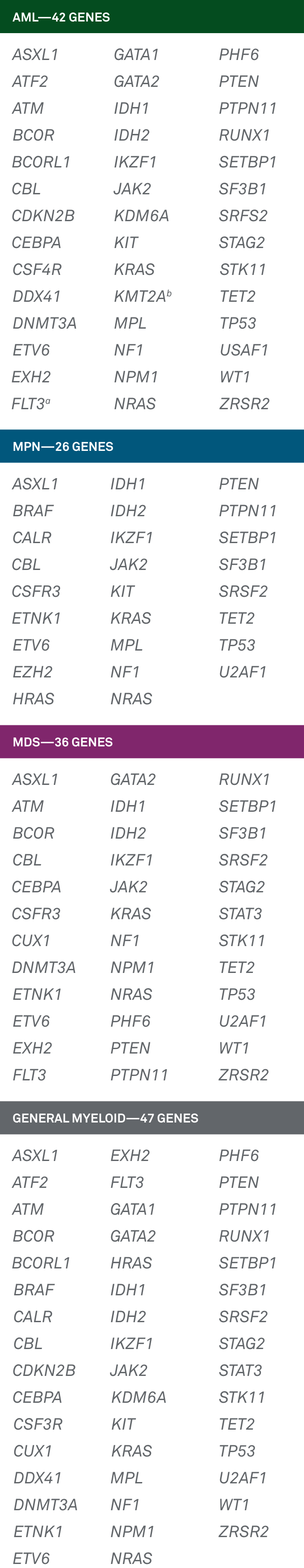
LeukoVantage® is an innovative selection of panels that provide confirmatory diagnosis, helping inform prognosis and therapeutic indications for:
- Acute myeloid leukemia (AML)
- Myeloproliferative neoplasms (MPN)
- Myelodysplastic syndrome (MDS)
- General Myeloid Panel
LeukoVantage panels utilize the latest in NGS technology, so you and your patients can know more, sooner, and make more informed treatment decisions.
Built on recent guidelines
LeukoVantage is a panel that offers physicians testing solutions for these disease states: acute myeloid leukemia (AML), myeloproliferative neoplasms (MPN), myelodysplastic syndrome (MDS) and other myeloid neoplasms. Based on a comprehensive literature review and updated guidelines for gene inclusion from the World Health Organization and National Comprehensive Cancer Network®, the analyzed genes can help physicians make a more informed diagnosis and more closely monitor disease progression and their patients’ response to treatment.1-4
These 4 panels can help:
- Classify acute myeloid leukemia (AML)
- Aid in diagnosis of myeloproliferative neoplasms (MPN)
- Make an early assessment of myelodysplastic syndrome (MDS)—detects mutated genes in early MDS when cytogenetics is uninformative3
A comprehensive series of 4 panels that interrogate genes that are guideline-indicated as relevant to myeloid diseases
The LeukoVantage Myeloid Mutation panels utilize NGS technology to reliably detect mutation levels down to 5% allele levels, single nucleotide variants, small insertions, and deletions.
a Internal tandem duplications and tyrosine kinase codon 835/836 mutations
b PTD: partial tandem duplication detected by a long-range PCR-based method
Enhanced reporting and ordering for more informed patient care
Specimen requirements
c 1 week if collected in heparin tubes
References
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. doi:10.1182/blood-2016-03-643544
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Acute Myeloid Leukemia, Version 1.2018—February 7, 2018. Accessed July 15, 2018. https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Myelodysplastic Syndromes, Version 2.2018—February 15, 2018. Accessed July 15, 2018. https://www.nccn.org/professionals/physician_gls/pdf/mds.pdf
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Myeloproliferative Neoplasms, Version 2.2018—September 7, 2017. Accessed July 15, 2018. https://www.nccn.org/professionals/physician_gls/pdf/mpn.pdf
- Rosenthal SE, Gerasimova A, et al. Evaluation of the 42-Gene Next Generation Sequencing Panel for Detection of Acute Myeloid Leukemia Mutations for Clinical Use. Abstract presented at ASCO Annual Scientific Meeting: 2019; Chicago, IL